 Graphical abstract of my 2013 thesis proposal on the biology of Blimp1 (PRDM1) in radiogenic cancer
Graphical abstract of my 2013 thesis proposal on the biology of Blimp1 (PRDM1) in radiogenic cancer
For my thesis proposal for the Committee on Cancer Biology at University of Chicago in 2013, I proposed a series of in vitro and in vivo experiments to elucidate the cell signaling of Blimp1 (PRDM1) following induction by ionizing radiation (IR). IR exposure durng cancer radiotherapy damages healthy tissue and risks causing radiogenic second cancers as a late side effect of treatment, particularly for pediatric cancers. A previous GWAS study implicated a SNP causing differential expression of Blimp1 and differential induction of Blimp1 expression as an extremely powerful predictor of secondary breast cancer following radiotherapy for pediatric cancers. Individuals with the genotype associated with increased Blimp1 expression after IR exposure had a significantly lower risk of secondary cancer compared to individuals with the genotype associated with non-inducible Blimp1 expression following IR exposure. My goals was to elucidate the signaling pathways induced by Blimp1 in response to radiotherapy in order to clarify why Blimp1 expression in that context might be protective against future cancer.
I successfully obtained PhD candidacy with this proposal. These documents support my ability to design independent research, perform preliminary studies, and communicate science.
I ultimately did not complete this project due to a toxic work environment in my lab. With the support of the dean of the Biological Sciences Division, I sought a new lab, but my advanced standing in the program complicated funding for my prospective mentors. I chose to leave with my master’s degree and focus primarily on biology education while working part-time in research. Ultimately I decided to return to a career more engaged in research, leading me back to graduate school.
Abstract
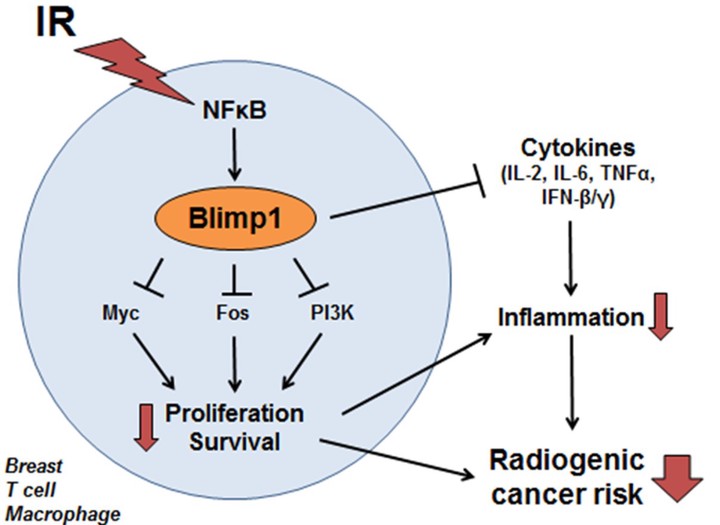
Radiogenic cancer is a common consequence of ionizing radiation (IR) exposure from radiation therapy (RT). Genetic variants in the Blimp1 (PRDM1) locus modulate the risk of second cancers following RT. Preliminary data suggest Blimp1 is activated by IR and protects against radiogenic cancer. I intend to determine the cellular and molecular mechanisms by which Blimp1 protects against radiogenic cancer. Blimp1 is a pleiotropic transcriptional repressor with diverse roles in regulating stress responses in the immune system and epithelial cells. I hypothesize that IR-mediated Blimp1 activation protects against breast tumorigenesis by modulating stress response pathways in breast cells and/or immune cells. First, I will determine the mechanism of Blimp1 activation by IR, both by determining the cell types in which Blimp1 is activated by IR and by testing whether IR activates Blimp1 via NFκB. Then, I will determine the cellular and molecular consequences of IR-mediated Blimp1 activation. In response to stress, Blimp1 often reduces proliferation and survival in inflammatory and epithelial cells. Consequently, I will test whether IR-mediated Blimp1 activation modulates cell proliferation and survival. I will determine whether IR-activated Blimp1 represses a set of candidate Blimp1 target genes that regulate proliferation, survival, and inflammation. Last, I will establish whether Blimp1 protects against radiogenic cancers in vivo in two mouse models of radiogenic cancer. I will knock down Blimp1 in p53-/- primary mouse mammary epithelial cells, xenograft these cells into cleared mammary fat pads, then irradiate the mice and determine whether Blimp1 knockdown reduces breast tumor latency and therefore increases radiogenic cancer risk. In addition, I will test whether Blimp1 more generally protects against a range of radiogenic cancers by irradiating p53+/- mice in a Blimp1 wild-type or heterozygous background to determine whether Blimp1 deficiency reduces the latency of radiogenic cancers in a variety of tissues. If successful, I will elucidate the mechanisms by which IR activates Blimp1, the cellular and molecular consequences of IR-mediated Blimp1 activation, and whether Blimp1 indeed protects against radiogenic cancers in vivo. This proposal is significant because Blimp1 activity could be modulated to protect individuals undergoing RT from radiogenic cancers, and because Blimp1 is a common component of stress response and inflammatory processes and these findings could have wider implications for normal tissue biology and protection against other stress-induced cancers.